Reimbursement pathways are complex and lengthy. For many technologies, harsh reality crashes the expectations of obtaining access early or getting fast uptake. For many minimally invasive expensive medical technologies, transcatheter aortic valve implantation (TAVI) is a very interesting case study of getting access to market. With this post, we will open a series of articles about the reimbursement journey for TAVI in different European countries. In this post, we will focus on developments of procedure coding for TAVI since its CE mark in Europe for the first two products in 2007.
Specific procedure coding is essential to secure reimbursement, as it is used in combination with diagnosis code and other parameters to allocate the case into specific diagnosis-related group (DRG), which drives reimbursement in majority of EU geographies. Some countries (like England and Italy) do not have very detailed nomenclatures, so combination of codes or unspecific codes are often used to describe procedures. In Belgium and France, each procedure code has a monetary value, so the process to establish the code is lengthy and connected to review by payers / technology assessors.
The first TAVI devices were CE marked in 2007. European countries had different speed of introducing procedure codes for TAVI (see the graph). However, in all countries, availability of procedure code did not automatically lead to reimbursement (Belgium, France) or appropriate reimbursement (majority of other countries). Changes of reimbursement system, initiated by the creation of procedure code, were required.
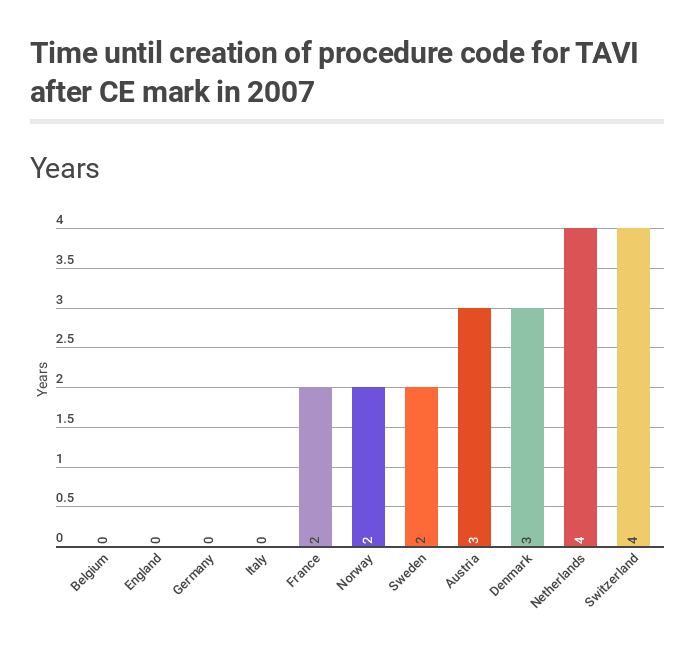
Timing for creation of the first procedure codes was the following:
- In Austria, the first provisional (innovative, NUB) code for TAVI was established in 2010
- In Belgium, procedure code was already in place since 1991, however there was no material code for the technology. The first material code was established only in 2014
- In Denmark, a specific procedure code was created in 2010
- In England, combination of OPCS codes, required to describe the procedure, was available at the time of CE mark
- In France, CCAM codes for TAVI were created in 2009
- In Germany, OPS code for transluminal access was established the year before CE mark, in 2006. In 2008, it was divided into codes for transluminal and transapical access. In 2014, further split was made
- In Italy, the ICD-9-CM coding system from 2002 was in place at the time of CE mark of TAVI. Unspecific procedure codes, which were lately recommended by authorities and clinical societies to code TAVI were in place at the time of CE mark in 2007
- In the Netherlands, the code for transluminal access was created in 2009, it was followed by the code for transapical access in 2011
- In Norway, the codes were established in 2009
- In Sweden, the codes were established in 2009
- In Switzerland, the CHOP codes for TAVI were introduced in 2011 (nationwide DRG system was implemented in 2012)
So, in general countries were relatively quick in establishing the procedure codes for TAVI, however it did not automatically translate into reimbursement or appropriate reimbursement.
Stay tuned and follow further story about establishment of formal reimbursement, commissioning for TAVI and evolution of uptake of procedure in Europe. Subscribe to our newsletter, so you do not miss an important information!
