MTRC has released reimbursement report for transcatheter aortic valve implantation in 11 European countries. Report includes information about procedure coding, payment mechanisms, reimbursement tariffs and policies for TAVI.
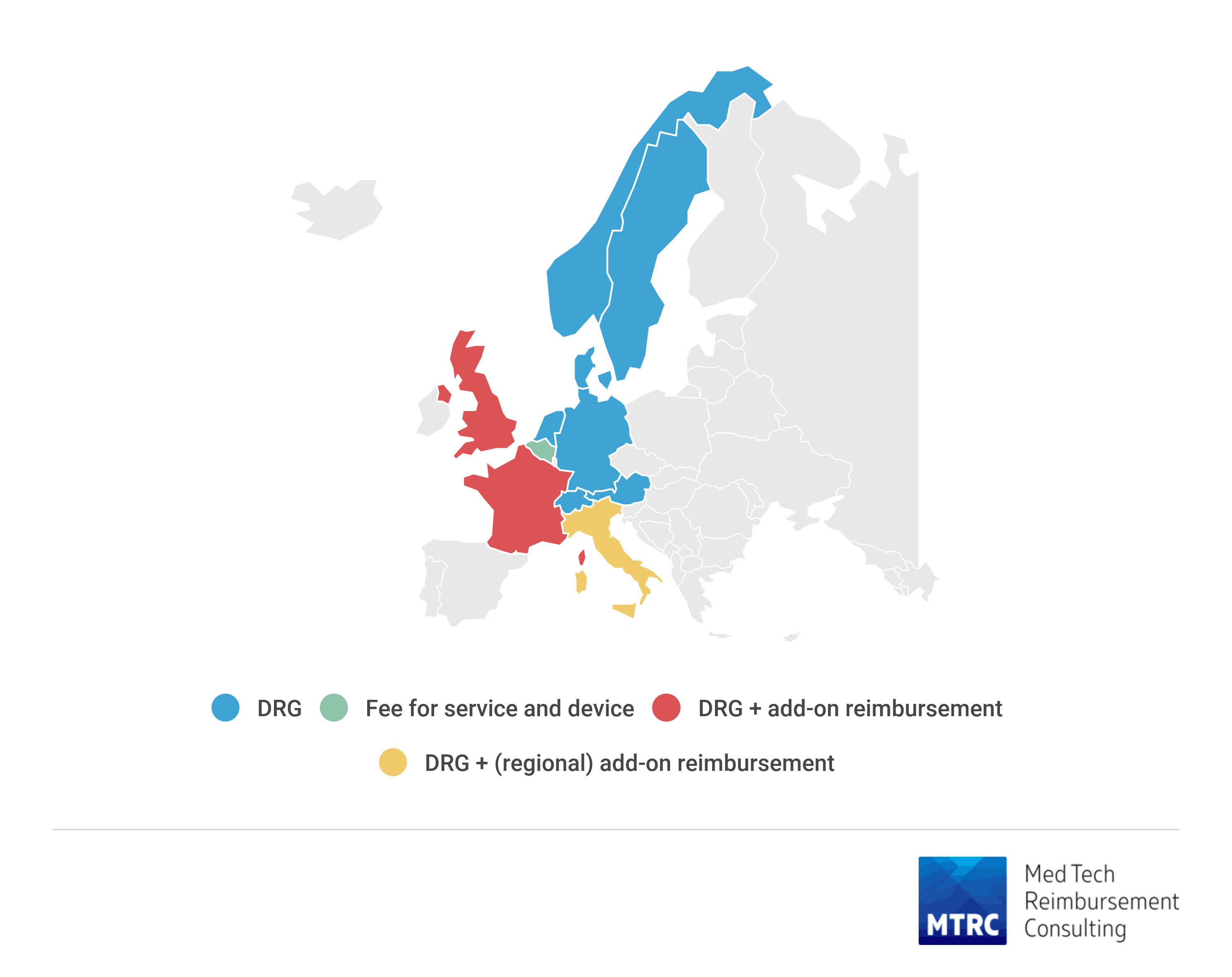
TAVI is well established technology with reimbursement available in all studied European geographies. The main payment models are diagnosis-related group (DRG) for entire hospitalization, add-on reimbursement (England, France, Italy in some regions) and fee for procedure and material (Belgium). All geographies, but Italy, have specific procedure codes for TAVI. Brand-specific reimbursement is only available in Belgium and France, while in other countries reimbursement is established for the class of devices.
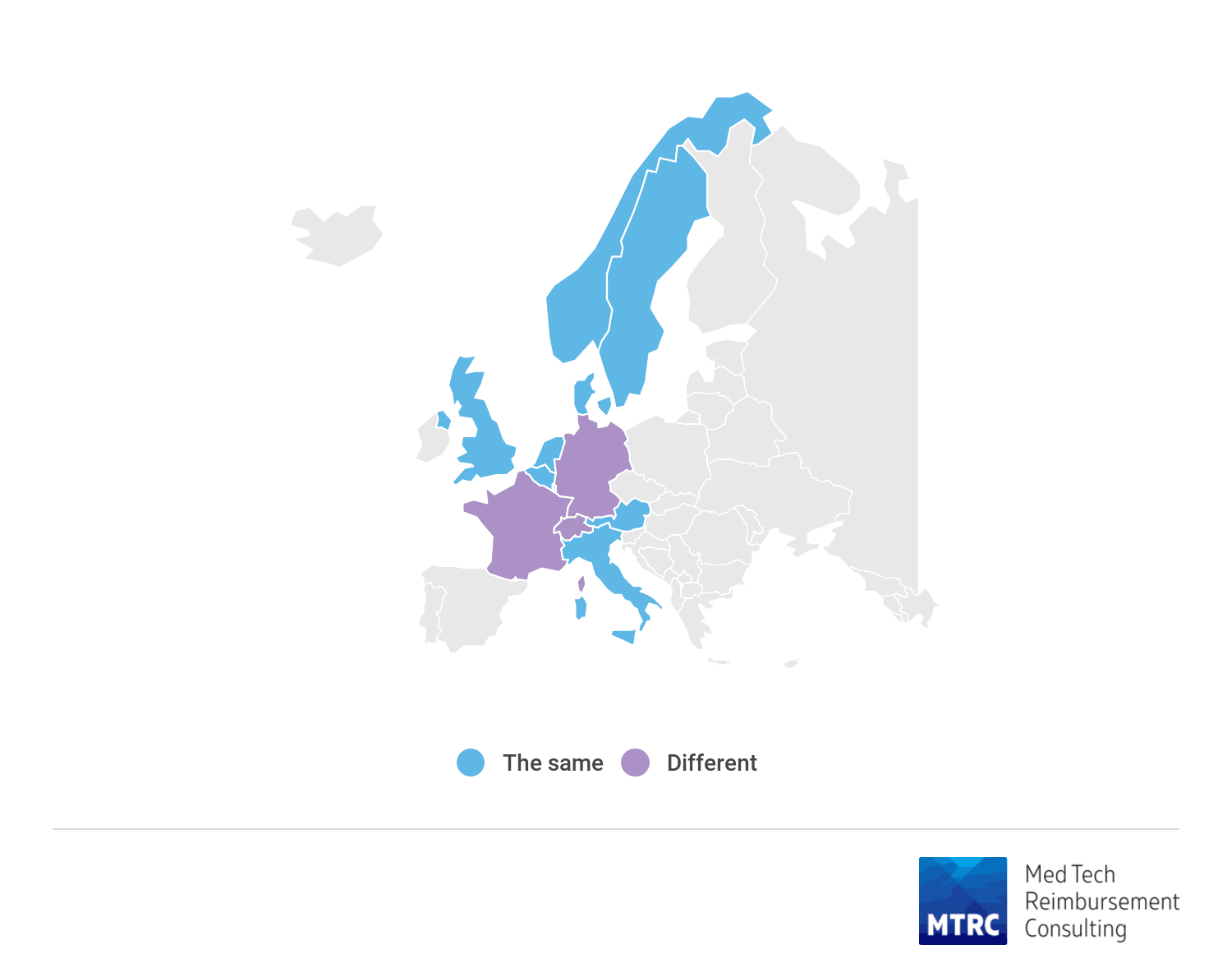
Reimbursement differs for different types of access for procedure only in France, Germany and Switzerland. In Germany and Switzerland procedure with transapical access has higher reimbursement.
Complications or stroke specifically do not impact allocation to DRG and reimbursement level in any of studied countries, with exception of France.
Almost every country has implemented certain restrictions to provision of TAVI, which are limited to inoperable or operable patients at high surgical risk in majority of studied geographies.
Report is available for order from this page. It is also possible to request an extract from the report for review.
