Minimally invasive (transapical) mitral valve replacement is an emerging technology. While this technology will require a dedicated effort to get acceptance from medical community and payers in most of the geographies it will also face a challenge of establishing an appropriate reimbursement mechanism.
MTRC has recently completed a reimbursement overview of this procedure in 11 European countries. Based on the results of the analysis, we have developed a map with summary of reimbursement situation for the procedure.
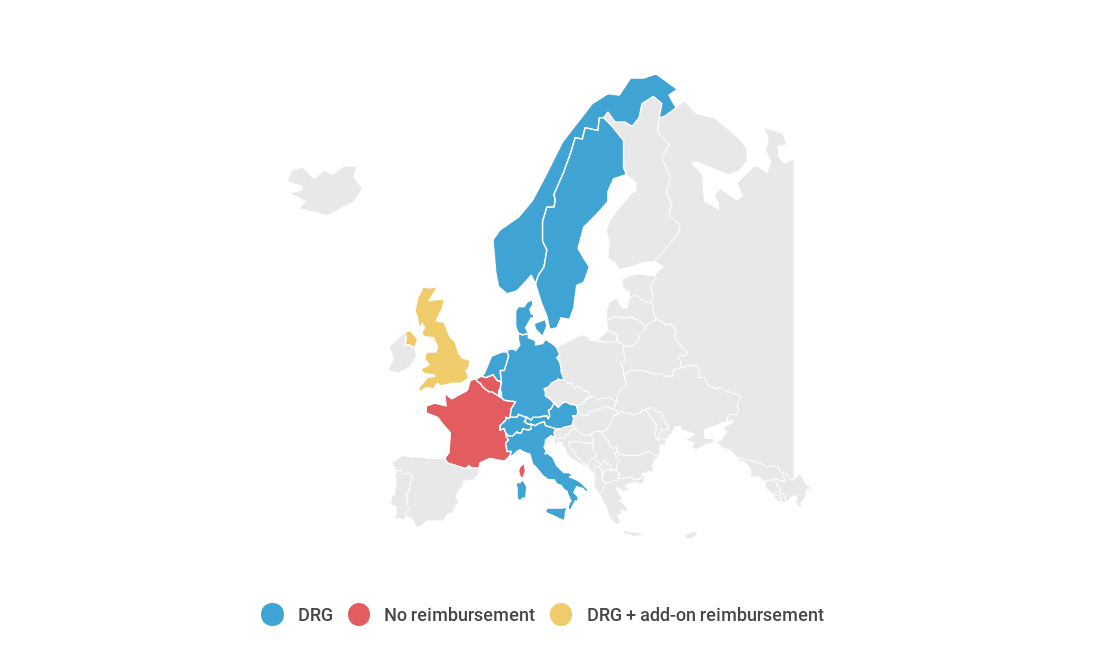
The procedure can be coded in Austria, Denmark, England, Germany, Italy, Netherlands, Norway, Sweden and Switzerland, so the payment mechanism is in place. In countries where the procedure is not coded, it cannot be reimbursed.
In countries where the procedure is coded, the main payment mechanism is via DRG. In England, add-on reimbursement is available via the High Cost Device List.
The presence of procedure coding and reimbursement does not mean that payers are willing to accept the technology and pay for it. While the decision to use the technology is up to the hospitals in the countries where the procedure has been coded, there remains a strict funding barrier for use of the technology in Austria, England and Norway.
Are you interested in buying the report? Please, proceed to the report’s page.
